About us
The story began back in 1956: At that time the founders of Immuno in Austria, Dr. Eibl and Dr. Schwarz, successfully cooperated in the development of a polio vaccine. And twenty years later, in 1976, Immuno started production of the vaccine against tick-borne encephalitis (TBE).
The other story began in 1990: North American Vaccines was founded. And in 1998, this company was the first independent vaccine manufacturer in more than a decade to enter the US pediatric vaccines market with its first product, a diphteria, tetanus, acellular pertussis (DTaP) vaccine. Only two years later a group C meningococcal conjugate vaccine , was launched in the UK.
At Genevac Vaccines, we are on our way to be a leading force in the vaccines market driven by the promises of a better future.

What is tick-borne encephalitis (TBE)?
Tick-borne encephalitis (TBE) is a viral infection involving the central nervous system. The TBE most often manifests as meningitis, encephalitis or meningoencephalitis. Myelitis and spinal paralysis also occur. In about one third of cases sequelae, predominantly cognitive dysfunction, persist for a year or more.
The number of reported cases has been increasing in most countries. TBE is posing a concerning challenge to Europe, as the number of reported human cases of TBE in all endemic regions of Europe has increased by almost 400% within the last three decades.
The tick-borne encephalitis is known to infect a range of hosts including ruminants, birds, rodents, carnivores, horses, and humans. The disease can also be spread from animals to humans, with ruminants and dogs providing the principal source of infection for humans.

TBE Virus
(TBEV), like yellow fever, Japanese encephalitis, and dengue virus, is a member of the genus flavivirus belonging to the family Flaviviridae. Due to the specific route of transmission by infected ticks (e.g., TBE virus, Louping ill virus) or mosquitos (e.g., yellow fever virus, dengue viruses, West Nile virus and Japanese encephalitis virus) most of the flaviviruses are so-called arboviruses .
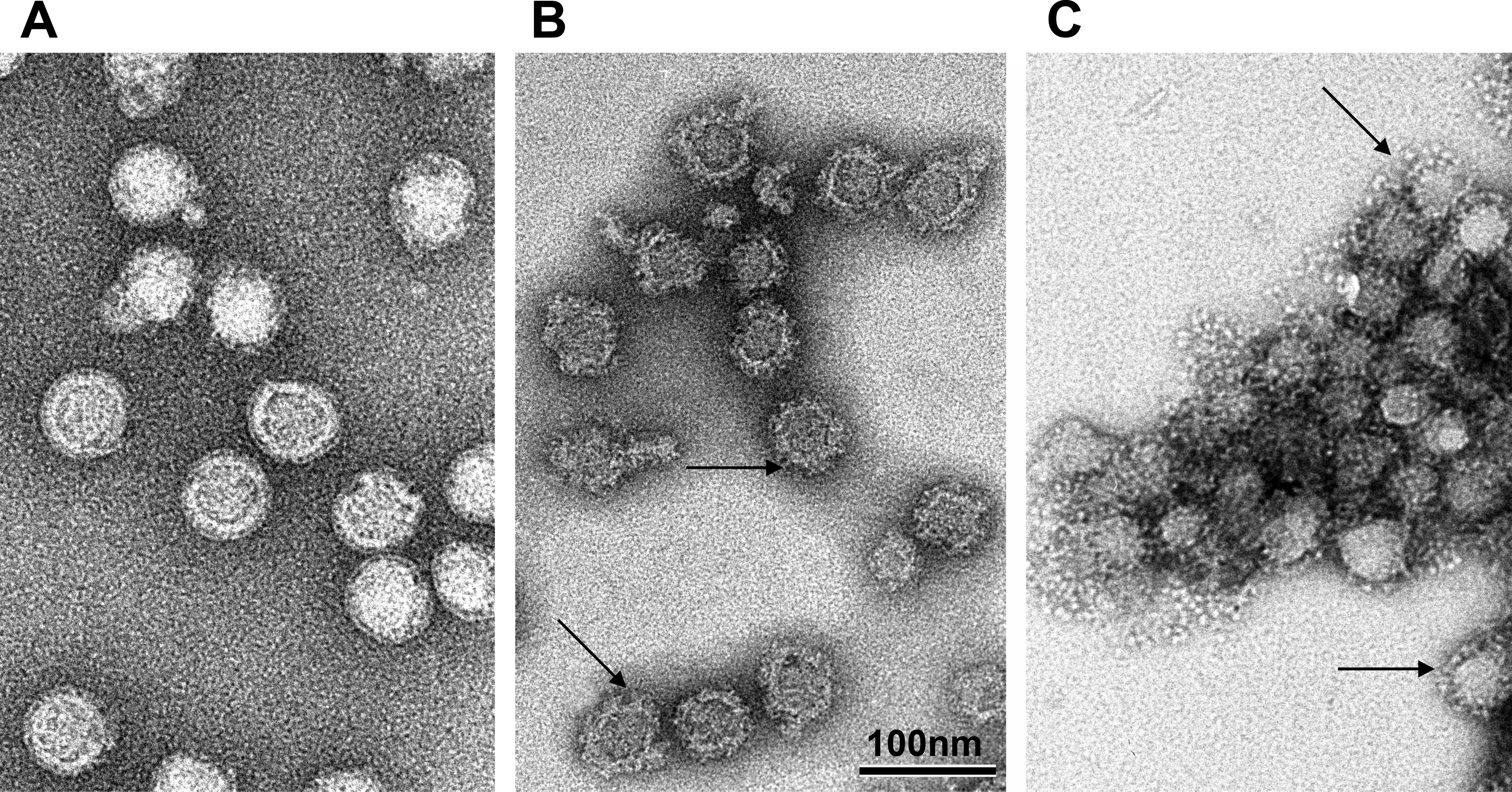
Flaviviruses are spherical, lipid-enveloped RNA viruses with a diameter of approximately 50nm, which contain of only three different structural proteins, i.e. the proteins C (capsid), M (membrane) and E (envelope). The protein C is the only protein component of the capsid, which encloses a positive-stranded RNA approximately 11,000 nucleotides in length.This RNA codes for the three structural proteins, as well as a set of 7 non-structural proteins required for it replication in the cell.
The proteins E and M are incorporated in the viral membrane. Glycoprotein E, the main component of the viral surface, is responsible for the formation of neutralising antibodies and the induction of protective immunity. By isolating a soluble, crystallizable form of the TBEV protein E it was possible to elucidate its three-dimensional structure using X-ray diffraction analysis.
Structural analysis has shown that protein E, unlike the envelope proteins of many other lipid-enveloped paricules, does not form spike-like projections, but is aligned parallel to the viral surface.

Clinical course and manifestations
The typical course of TBE is diphasic in at least two-thirds of patients, and can be described as follows. The incubation period may last between 2 and 28 days, but on average is 7 days. The first stage, which may last for 2 to 8 days, corresponds with the viremic phase. It is associated with non-specific systemic signs and symptoms such as fatigue, headache, aching back and limbs, nausea, and general malaise; in most cases the temperature is rising to 38°C or higher. Sometimes exceptionally high initial temperature may occur, rising above 40°C. An afebrile interval follows the first stage of TBE, and lasts 1 to 20 days. During this time patients are usually free of symptoms. Another sudden rise of temperature to high values marks the beginning of the second stage.

Not all individuals infected with TBE go through the entire course of the disease. In approximately two thirds of infected individuals the infection remains either silent, though viremia can be demonstrated, or the patients show the clinical picture of the initial stage of TBE, but then the symptoms subside without developing into the second stage.
About one third of those symptomatic with TBE proceed into the second stage of the disease after the TBEV has spread to the CNS. 50–77% of these patients go through the typical biphasic course of the infection. In the remaining 23–50% the infection is in-apparent during the first stage, and the onset of clinical illness coincides with the beginning of the second phase of the disease.
The course of disease with the Far-Eastern variety clinically differs from the European form. The onset of illness is more often gradual than acute with a prodromal phase including, fever, headache, anorexia, nausea, vomiting and photophobia. These symptoms are followed by a stiff neck, sensorial changes, visual disturbances, and variable neurological dysfunctions, including paresis, paralysis, sensory loss and convulsions. In fatal cases, death occurs within the first week after onset. The case-fatality rate is approximately 20% compared to 1–2% for the European form but these figures may be biased by the different standards of medical treatment available in Western Europe and eastern regions. It is supposed that, in contrast to the European form the disease caused by the Far-Eastern variety is more severe in children than in adults. Neurological sequelae occur in 30–80% of survivors, especially residual flaccid paralyses of the shoulder girdle and arms. Little information is available on the virulence of the recently described Siberian subtype with respect to the course of disease in humans. However, animal studies have demonstrated that the limited number of Siberian subtype strains studied have higher virulence in mice than Far-Eastern strains.

Mixed Infections
Co-infections involving various combinations of pathogens are frequently described, and some tend to be particularly severe. Diseases caused by mixed TBE -Borrelia infections have already been revealed in several countries of Central Europe. Co-infection with Borrelia burgdorferi is reported in about 15% of patients on the basis of seropositive results in serum and/or CSF. In the majority of patients with concomitant infection the clinical features at presentation were characteristic of, or consistent with, TBE. It is suggested that in confirmed cases of tick-encephalitis in patients with acute lymphocytic meningitis or meningoencephalitis, originating in TBE and Lyme borreliosis endemic regions, an additional infection with Borrelia should be considered since – if present – the latter can be successfully treated with antibiotics. There is some information in the literature that co-infection with B. burgdorferi sensu lato might contribute to a more severe course of TBE. Similar findings of mixed infections are described in children, most commonly TBE and Lyme borreliosis. Double infections occur more frequently in areas where I. persulcatus ticks are abundant. A study from the Czech Republic demonstrated that TBE/HGE (human granulocytic ehrlichiosis) co-infections can also be encountered in Central Europe.

Prognosis
Hospitalization is usually required for about three weeks; however, in severe cases it may last much longer, sometimes even several years. With rising age of the patient, especially in persons above 60, TBE increasingly takes a severe course, leading to paralysis and sometimes ending in death. The results of laboratory examinations seem to have little predictive value for the severity of the course of the disease, excluding CSF cell counts, which may indicate the intensity of CNS infection, and correlate with the severity of the disease. Similarily, TBE antibody concentration as detected by ELISA does not correlate with the outcome of the infection, whereas the neutralizing antibody titer – together with the CNS cell count – seems to be more predictive for the clinical outcome. At onset of disease, the presence of a low concentration of neutralizing antibodies in serum, and a high cell count in the CSF might indicate an unfavourable course of TBE.

Severity of TBE in relation to other CNS viral diseases
According to a study conducted in 1958, the proportion of TBE in the total number of CNS viral diseases in Austria was 56%. Thus, before the start of the vaccination program, it was the most important and most frequent disease of this type in adults – with several hundred cases being reported each year.
In Switzerland, in 1981 TBE ranked fourth among viral infections of the central and peripheral nervous system, only picorna, mumps and Varicella zoster infections were more frequent. In some cantons, however, it was the most frequent cause of CNS diseases. In Germany, TBE accounts for up to 50% of all viral diseases, and in Lithuania TBE accounts for more than half (53%) of all CNS infections.
Diagnosis
The diagnosis of TBE is based on :
Epidemiological information: stay in a TBE risk area, facultative history of a tick bite
Clinical data: uncharacteristic and usually not sufficient for diagnosis
Demonstration of TBE-specific IgM and IgG antibodies in serum (adequate evidence of infection) and CSF

Laboratory Diagnosis
The actual diagnosis of TBE must be established in the laboratory because of the non-specific clinical features it presents. The laboratory results have no influence on the therapy of TBE, and mainly serve to differentiate a TBEV infection from other causes of meningoencephalitis, which may require special treatment.The method of choice is the demonstration of specific IgM and IgG serum antibodies by enzyme-linked immuno-sorbent assay (ELISA). As the symptoms affecting the CNS are not usually observed until two to four weeks after the tick bite, the antibody test is nearly invariably positive at the time of admission to hospital. Soon after infection IgM antibodies are more specific, while later, IgG antibodies are more reactive. A recent infection can be established by the qualitative determination of IgM. Specific IgG antibodies and rheumatoid factors do not interfere with the test. However, in cases of other flavivirus contacts (e.g. vaccinations against yellow fever or Japanese encephalitis; dengue infections) the performance of a neutralization assay (e.g. RFFIT, rapid fluorescent focus inhibition test) is necessary for assessing immunity due to the interference of flavivirus cross-reactive antibodies in ELISA and hemagglutination inhibition test.The high cross-reactivity rate of yellow fever and TBE antibody-positive sera in dengue particul antibody assays should be taken into account in the interpretation of laboratory tests for the diagnosis of flavivirus infections, and when undertaking seroepidemiological surveys.Neutralization tests – like RFFIT – require handling infections, which makes the test cumbersome, costly and only available in highly specialized laboratories. IgG antibodies are detected by qualitative methods, e.g. establish the prevalence of TBE in the population or in a group of patients, or follow seroconversion after active immunization and control of humoral immune status.

Differential Diagnosis
Fever, headache and meningism associated with signs of inflammation in serum (leukocytosis, elevation of the sedimentation rate and of C-reactive protein), and predominance of neutrophilic cells over lymphocytes in the CSF are main findings in patients with TBE, but are also highly indicative of bacterial meningitis. Consequently, most patients are treated with antibiotics – at least until the TBE serology is found to be positive.Thus, many viral and bacterial infections have to be considered in the differential diagnosis of TBE.Lyme disease (lyme borreliosis) has been recognized as the most frequent vector borne disease in mild climate areas, and has to be included in the differential diagnosis of TBE. Its causative agent, Borrelia burgdorferi sensu lato complex (B. burgdorferi sensu stricto, B. garinii and B. afzelii), is transmitted by ticks and other arthropods. In our part of the world, its incidence is higher than that of TBE. Contrary to TBE, the various stages and the manifestations of lyme borreliosis occur facultatively; transitions may be indistinct. High-dose administration of penicillin, cephalosporin, macrolide or doxycycline is the therapy of choice.Acute human granulocytic ehrlichiosis (HGE) is an emerging tick-borne disease, which should now be included in the differential diagnosis of febrile illnesses occurring after a tick bite in Europe. HGE is caused by Ehrlichia phagocytophila (Anaplasma), gramnegative intracellular bacteria infecting white blood cells. Comparing the clinical signs and laboratory findings of adult patients with proven acute HGE with that of patients in the initial phase of tick-borne encephalitis shows that the duration of fever in the initial phase of TBE is shorter (median 4 days vs. 7 days in patients with acute HGE). Clinical signs including chills, myalgia and arthralgia, and laboratory findings e.g. elevated values for lactate dehydrogenase and C-reactive protein direct towards a diagnosis of acute HGE rather than the initial phase of TBE.

Therapy
No specific therapy for TBE is known so far. Since there is no specific treatment targeting the TBEV itself, symptomatic treatment of patients with TBE is required. Strict bed rest for at least ten days is imperative. In many Austrian hospitals, encephalitis patients are referred to intensive care units for continuous surveillance as a precaution. Only when their temperature is down to normal, and neurological symptoms have subsided, the patient may start to leave bed briefly for washing and using the toilet. For another 1 to 2 weeks predominant bed rest is recommended to avoid complications.
Maintenance of the water and electrolyte balances, sufficient caloric intake, and the administration of analgesics, vitamins, and antipyretics constitutes the most important lines in the clinical management of patients. Physiotherapy of paralysed limbs is essential to prevent muscular atrophy.
Man is the dead-end host in the natural transmission cycle of TBE. As man-to-man transmission of the TBEV has never been observed, there is no need to isolate TBE patients.

Genevac TBE vaccine
is today licensed in all countries where this disease is endemic and it is helping to reduce the morbidity and mortality of this potentially life-threatening disease. In Austria, previously one of the most severely affected countries, an extensive public health effort has contributed effectively to reaching an overall vaccination rate of 84%, with a corresponding decrease in the number of hospitalized cases of TBE.
Meningococcal infection
Meningococcal infection is an important cause of morbidity and mortality worldwide. It is a contagious disease caused by the Gram-negative diplococcus Neisseria meningitidis that is divided in at least 12 serogroups. Transmission occurs by direct contact or droplets from the nose or throat (sneezing or coughing) of infected persons. It is the only form of bacterial meningitis that causes epidemics. Even in countries with well organized health care systems 5-10% of patients die of meningococcal disease and about 20% of survivors will suffer severe neurological dysfunctions, including deafness, palsies, seizures, and mental disorders.

Product information

A Novel Group C Meningococcal Conjugate Vaccine
Conjugate vaccines represent a new generation of vaccines in which the body’s immune system elicits a stronger, longer lasting antibody response across a wider age range than non-conjugate vaccines. The novel group C meningococcal conjugate vaccine is composed of specific fragments of the meningococcal polysaccharide coat linked to a non-toxic tetanus toxoid. The end result is a higher level of protection against infectious diseases. This long-term protection in children enables conjugate vaccines to be used in routine child immunization programs.The populations at risk from bacterial meningitis span all age categories from infants to adults but children from 3 months to 5 years of age and adolescents (15-18) are most susceptible. In Europe and North America, group C meningococcus is a leading cause of meningococcal infection, frequently resulting in permanent brain damage and death. In the United States, meningococcal C infections occur at an estimated rate of one to three cases out of 100,000 people each year -- primarily children and young adults -- with a fatality rate of approximately 15 %.
